Theme: Focused on all research activities for optimal use and safety of medication
Pharmacoepidemiology Congress 2018
Conference Series Ltd is delighted to extend our warm welcome to you all to participate in the 12th International Conference on Pharmacoepidemiology and Clinical research, scheduled at Seoul, South Korea during October 17-18, 2018. , with the theme "Focused on all research activities for optimal use and safety of medication”.
Why to Attend???
12th International Conference on Pharmacoepidemiology and Clinical research October 17-18, 2018 at Seoul, South Korea is organised aiming to bring together leading academic professors, scientists, researchers and research scholars to exchange and share their experiences and research results on all aspects of Pharmacoepidemiology and Clinical research. It also provides a premier interdisciplinary pavement for Pharma, Biotech and Medical Device industries and to represent their company and discuss the most recent innovations, trends, and concerns as well as practical challenges encountered and solutions adopted in the fields of Pharmacoepidemiology and Clinical trials and clinical research.
Pharma-epidemiology congress includes a wide range of Keynote presentations, Oral talks, Poster presentations, Symposia, Workshops, Exhibitions and extensive networking and B2B interactions.
ConferenceSeries Ltd Organizes 3000+ Global Events Every Year across USA, Europe & Asia with support from 1000 more scientific societies and Publishes 700+ Open access journals which contains over 100000 eminent personalities, reputed scientists as editorial board and organizing committee members.
Mark your dates to join the critical discussions and meet the experts at Pharma-epidemiology congress, Kuala Lumpur, Malaysia to enleash Advanced Clinical research and compliance in Pharmacoepidemiologic studies in the arena of Pharmacoepidemiology.
Who Should Attend ???
Directors/Senior Directors/Executive Directors and Vice Presidents/Senior Vice Presidents/Executive Vice Presidents and Heads/Leaders/Partners of
CROs and CMOs
Clinical Research Sites
Pharma/Biotech and Medical Device industries
Hospitals, Associations
Medical Directors, Principal Investigators, Methodologists, and other clinical research professionals along with Academicians: University Faculties like Directors, Senior Professors/Assistant Professors/ Associate Professor, Research Scholars, scientists who are related to clinical and medical research.
Clinical/Pharmaceutical and biotech industry professionals with responsibilities in:
Clinical Research & Development
Clinical Design/ Protocol Design/ Clinical Strategy
Global Clinical Operations/ Clinical Outsourcing
Biostatistics/Data management
Patient Recruitment/Enrollment
Clinical Trial Management/Clinical Trial Supplies
Regulatory Affairs
Conference Series Ltd is delighted to extend our warm welcome to you all to participate in the 12th International Conference on Pharmacoepidemiology and Clinical research,, scheduled at Seoul, South Korea during October 17-18, 2018. , with the theme "Focused on all research activities for optimal use and safety of medication”.
Experts Meeting to join the Global scientific influencers from Asia, Middleeast, Europe, USA(America) and Africa at Pharmacoepidemiology and Clinical research Congress, organised at Seoul, South Korea October 17-18, 2018. with a main theme “Focused on all research activities for optimal use and safety of medication”.
Pharmacoepidemiology Congress includes a wide range of Keynote presentations, Oral talks, Poster presentations, Symposia, Workshops, Exhibitions and extensive networking and B2B interactions.
Pharmacoepidemiology is an extensive area of science which involves uses and effects of drugs in larger arena of population. Pharmacoepidemiology is a bridge of science which connects both pharmacology and epidemiology enhancing the informative study on topics apparently related to Pharmacoepidemiology, Pharmacokinetics, Adverse drug reactions, Public health, Epidemiologic methods, Medication adherence and Drug safety, Pharmacovigilance, effects of genetic variation on drug effect, duration-response relationships, clinical effects of drug-drug interactions. This topic serves as a best way for Researchers to express their findings as research presentations on an array of Pharmacoepidemiology and Drug Safety. The main aim of Pharmacoepidemiology within the industry is to protect patients from unnecessary medication hazards by identifying the adverse effects, elucidating pre-disposing factors, refuting false safety signals and quantifying risk in relation to benefit. Clinical research is the branch of science that involves the use of various medications, clinical therapeutics, medical and clinical devices, equipment and medical products for diagnosis and treatment of human disorders, diseases and patient safety.
12th Global Pharmacovigilance & Drug Safety Summit, July 9-10, 2018 Sydney, Australia; 9th World Congress on Bioavailability & Bioequivalence,March 19-21, 2018 Dubai,UAE; 5th Annual Congress on Chemistry in Drug Discovery & Designing ,April 16-17, 2018 Dubai ,UAE, 2nd World Heart Congress May 14-16, 20178 Tokyo Japan; 9th Asian Biosimilars Congress August 20-22, 2018 Tokyo Japan .
Related Associations or Societies:
International Society for Pharmacoepidemiology, Center for Drug Safety and Effectiveness, Center for Public Health and Human Rights, Center for Health Services and Outcomes Research, International Pharmaceutical Federation, International Society of Pharmacovigilance .
Pharmacotherapy also known as drug therapy is the use of a medical therapies (Surgical, Chemotherapy, Physical therapy, Radiation therapy) to treat disease. Drugs interact with receptors or enzymes present in cells, which promote healthy functioning and shave or cure illness. Drug Safety refers to the pharmacological science that ensures safety and is related to the collection, detection, assessment, monitoring, and prevention of adverse drug effects with pharmaceutical products. Safety monitoring in clinical trials involves the collection of adverse events, laboratory investigations including the planning, execution, data analysis and reporting of the safety information and details of the clinical examination of patients.
12th Global Pharmacovigilance & Drug Safety Summit, July 9-10, 2018 Sydney, Australia; 9th World Congress on Bioavailability & Bioequivalence,March 19-21, 2018 Dubai,UAE; 5th Annual Congress on Chemistry in Drug Discovery & Designing ,April 16-17, 2018 Dubai ,UAE, 2nd World Heart Congress May 14-16, 20178 Tokyo Japan; 9th Asian Biosimilars Congress August 20-22, 2018 Tokyo Japan .
Related Associations or Societies:
International Society for Pharmacoepidemiology, Center for Drug Safety and Effectiveness, Center for Public Health and Human Rights, Center for Health Services and Outcomes Research, International Pharmaceutical Federation, International Society of Pharmacovigilance .
Action of Drugs also known Pharmacodynamics. It refers to the study of Drugs, its biochemical, physiologic effects, the mechanisms and its action on the body or on microorganisms and other parasites within or on the body. It deals with drug action, which is the initial consequence of a drug-receptor interaction, and drug effect, which refers to the subsequent effects. The drug action of digoxin, for example: The inhibition of membrane Na+/K+-ATPase; the drug effect is augmentation of cardiac contractility. In this example, the clinical response may comprise of improved exercise tolerance.
12th Global Pharmacovigilance & Drug Safety Summit, July 9-10, 2018 Sydney, Australia; 9th World Congress on Bioavailability & Bioequivalence,March 19-21, 2018 Dubai,UAE; 5th Annual Congress on Chemistry in Drug Discovery & Designing ,April 16-17, 2018 Dubai ,UAE, 2nd World Heart Congress May 14-16, 20178 Tokyo Japan; 9th Asian Biosimilars Congress August 20-22, 2018 Tokyo Japan .
Related Associations or Societies:
International Society for Pharmacoepidemiology, Center for Drug Safety and Effectiveness, Center for Public Health and Human Rights, Center for Health Services and Outcomes Research, International Pharmaceutical Federation, International Society of Pharmacovigilance .
Clinical research is the branch of science that involves the use of various medications, clinical therapeutics, medical and clinical devices, equipment and medical products for diagnosis and treatment of human disorders, diseases and patient safety.
Related Pharmacoepidemiology Congresses | Clinical Research Meetings | Drug Safety events
12th Global Pharmacovigilance & Drug Safety Summit, July 9-10, 2018 Sydney, Australia; 9th World Congress on Bioavailability & Bioequivalence,March 19-21, 2018 Dubai,UAE; 5th Annual Congress on Chemistry in Drug Discovery & Designing ,April 16-17, 2018 Dubai ,UAE, 2nd World Heart Congress May 14-16, 20178 Tokyo Japan; 9th Asian Biosimilars Congress August 20-22, 2018 Tokyo Japan .
Related Associations or Societies:
International Society for Pharmacoepidemiology, Center for Drug Safety and Effectiveness, Center for Public Health and Human Rights, Center for Health Services and Outcomes Research, International Pharmaceutical Federation, International Society of Pharmacovigilance .
Clinical trials describe the distinct procedures followed in development of new drugs. These are the characteristic studies intended for the treatment of behavioural symptoms among the medical intervention and a health outcome. Clinical trials comprise patient data, disease mechanisms, therapeutic interventions, epidemiology, clinical medicine, preclinical and clinical pharmacology, immunology, toxicological studies. Clinical trials consist of experimental clinical trials scrutinizes the new treatment replacing the placebo or existing alternative treatments and safety methodology using data from observation of patients treated in clinical. Clinical data is the key feature translating basic research into medical care. The perfect conclusion of clinical trials is minimize risks, extend benefits and optimal use of an intervention of therapeutic drugs .
12th Global Pharmacovigilance & Drug Safety Summit, July 9-10, 2018 Sydney, Australia; 9th World Congress on Bioavailability & Bioequivalence,March 19-21, 2018 Dubai,UAE; 5th Annual Congress on Chemistry in Drug Discovery & Designing ,April 16-17, 2018 Dubai ,UAE, 2nd World Heart Congress May 14-16, 20178 Tokyo Japan; 9th Asian Biosimilars Congress August 20-22, 2018 Tokyo Japan .
Society for Clinical Trials, Association of Clinical Research, The Society of Clinical Research Associates, Clinical Research Society, American Association of Pharmaceutical Scientists, Association of British Pharmaceutical Industry .
Pharmacology is the study of interaction between substances with living organisms to produce a change in function. It deals with the research, discovery, and characterization of chemicals that show biological effects and the illumination of cellular and organism function in relation to these chemicals. If substances have medicinal properties, they are defined as pharmaceuticals. The Pharmacology encompasses mechanisms of drug action, drug composition and properties, interactions, toxicology, therapies, medical applications, and anti pathogenic capabilities.
12th Global Pharmacovigilance & Drug Safety Summit, July 9-10, 2018 Sydney, Australia; 9th World Congress on Bioavailability & Bioequivalence,March 19-21, 2018 Dubai,UAE; 5th Annual Congress on Chemistry in Drug Discovery & Designing ,April 16-17, 2018 Dubai ,UAE, 2nd World Heart Congress May 14-16, 20178 Tokyo Japan; 9th Asian Biosimilars Congress August 20-22, 2018 Tokyo Japan .
Related Associations or Societies:
International Society for Pharmacoepidemiology, Center for Drug Safety and Effectiveness, Center for Public Health and Human Rights, Center for Health Services and Outcomes Research, International Pharmaceutical Federation, International Society of Pharmacovigilance .
Pharmacoepidemiology is an extensive area of science which involves uses and effects of drugs in larger arena of population. Pharmacoepidemiology is a bridge of science which connects both pharmacology and epidemiology enhancing the informative study on topics apparently related to Pharmacoepidemiology, Pharmacokinetics, Adverse drug reactions, Public health, Epidemiologic methods, Medication adherence and Drug safety, Pharmacovigilance, effects of genetic variation on drug effect, duration-response relationships, clinical effects of drug-drug interactions. This topic serves as a best way for Researchers to express their findings as research presentations on an array of Pharmacoepidemiology and Drug Safety. Public health describes “the science and art of preventing disease, lengthening life and further human health through methodized efforts and informed choices of society, organizations, public and private, communities and individuals." It is concerned with threats to health based on population health analysis .The focus of public health intercession is to promote health and quality of life through obstruction and treatment of disease and other physical and mental health conditions. This is done through supervision of cases and health indicators, and through advancement of healthy behaviours
12th Global Pharmacovigilance & Drug Safety Summit, July 9-10, 2018 Sydney, Australia; 9th World Congress on Bioavailability & Bioequivalence,March 19-21, 2018 Dubai,UAE; 5th Annual Congress on Chemistry in Drug Discovery & Designing ,April 16-17, 2018 Dubai ,UAE, 2nd World Heart Congress May 14-16, 20178 Tokyo Japan; 9th Asian Biosimilars Congress August 20-22, 2018 Tokyo Japan .
Related Associations or Societies:
Society for Clinical Trials Association of Clinical Research The Society of Clinical Research Associates Clinical Research Society American Association of Pharmaceutical Scientists Association of British Pharmaceutical Industry International Society for Pharmacoepidemiology
Genetic epidemiology refers to “the role of genetic factors and genetic variations for determining health, disease in families and in populations and the interplay of such genetic factors with environmental factors. Genetic epidemiology seeks to derive a statistical and quantitative analysis of how genetics work in huge groups.
12th Global Pharmacovigilance & Drug Safety Summit, July 9-10, 2018 Sydney, Australia; 9th World Congress on Bioavailability & Bioequivalence,March 19-21, 2018 Dubai,UAE; 5th Annual Congress on Chemistry in Drug Discovery & Designing ,April 16-17, 2018 Dubai ,UAE, 2nd World Heart Congress May 14-16, 20178 Tokyo Japan; 9th Asian Biosimilars Congress August 20-22, 2018 Tokyo Japan .
Related Associations or Societies:
International Society for Pharmacoepidemiology Center for Drug Safety and Effectiveness Center for Public Health and Human Rights Center for Health Services and Outcomes Research International Pharmaceutical Federation International Society of Pharmacovigilance
Track 9:Drug Discovery and Drug Development:
Pharma compounds which were developed in the olden days were discovered and developed either by identifying the active ingredient from traditional remedies or by serendipitous discovery. But the advancements in the science and technology has made the job easier by controlling them at molecular and physiological level. During the phase of drug discovery main reason for the disease is identified and the possible pharmacological molecules are discovered. Later effect of that molecule on animals, humans is tested and if further improvements to be made, are performed. This involves many steps like identifying the reason for disease, lead identification, lead development, lead optimization, testing, and marketing.
12th Global Pharmacovigilance & Drug Safety Summit, July 9-10, 2018 Sydney, Australia; 9th World Congress on Bioavailability & Bioequivalence,March 19-21, 2018 Dubai,UAE; 5th Annual Congress on Chemistry in Drug Discovery & Designing ,April 16-17, 2018 Dubai ,UAE, 2nd World Heart Congress May 14-16, 20178 Tokyo Japan; 9th Asian Biosimilars Congress August 20-22, 2018 Tokyo Japan .
Related Associations or Societies:
Society for Clinical Trials Association of Clinical Research The Society of Clinical Research Associates Clinical Research Society American Association of Pharmaceutical Scientists Association of British Pharmaceutical Industry International Society for Pharmacoepidemiology
Databases contain professional assessment of the potential of drugs and permit the risk- profit analysis of medicinal products taking under consideration new and emerging information, within the context of additive data. These databases offer programming of alerts for fast cases, follow-up cases and reports submission to fulfil regulative timeline compliance. Data sources of pharmacoepidemiological databases are CPRD (Clinical Practice Research Data link), IMS Health Disease Analyser databases, PHARMO, Vigibase and Eudravigilance. Pharmacoepidemiology research studies may involve either data collected prospectively for the purpose of the particular study i.e. primary data, or data that were already collected for another purpose – as part of administrative records or patient health care , which is called secondary data.
12th Global Pharmacovigilance & Drug Safety Summit, July 9-10, 2018 Sydney, Australia; 9th World Congress on Bioavailability & Bioequivalence,March 19-21, 2018 Dubai,UAE; 5th Annual Congress on Chemistry in Drug Discovery & Designing ,April 16-17, 2018 Dubai ,UAE, 2nd World Heart Congress May 14-16, 20178 Tokyo Japan; 9th Asian Biosimilars Congress August 20-22, 2018 Tokyo Japan .
Related Associations or Societies:
International Society for Pharmacoepidemiology Center for Drug Safety and Effectiveness Center for Public Health and Human Rights Center for Health Services and Outcomes Research International Pharmaceutical Federation International Society of Pharmacovigilance
Clinical Pharmacy and Pharmacy Practice involves developing the professional roles of pharmacists and is the order of drug store which includes adding to the expert parts of drug specialists. It includes Disease-state management, Clinical drug interventions, Pharmacy professional development and pharmaceutical care, pharmaceutical compounding and health psychology, patient care, drug abuse prevention, prevention of drug interactions or minimisation of adverse events and drug incompatibility and community pharmacy.
12th Global Pharmacovigilance & Drug Safety Summit, July 9-10, 2018 Sydney, Australia; 9th World Congress on Bioavailability & Bioequivalence,March 19-21, 2018 Dubai,UAE; 5th Annual Congress on Chemistry in Drug Discovery & Designing ,April 16-17, 2018 Dubai ,UAE, 2nd World Heart Congress May 14-16, 20178 Tokyo Japan; 9th Asian Biosimilars Congress August 20-22, 2018 Tokyo Japan .
Related Associations or Societies:
Society for Clinical Trials Association of Clinical Research The Society of Clinical Research Associates Clinical Research Society American Association of Pharmaceutical Scientists Association of British Pharmaceutical Industry International Society for Pharmacoepidemiology
Pharmacovigilance plays a key role in all phases of clinical trials, including the planning, execution, data analysis and reporting of safety information and also bears an important responsibility for the expedited reporting of individual cases and safety updates required for regulatory authorities. The concept of Pharmacovigilance and its Significance enhances the impact of healthcare management on patient welfare and public health. Pharmacovigilance legislation gives an outlook on the rules and regulations to follow in Pharmacovigilance practice. The Role of Pharma industries in the improvement of Pharmacovigilance system is very crucial to maintain the safety data, detection and evaluation of drug safety signals through manual and medical devices reporting. Pharmacovigilance scope also deals as Eco Pharmacovigilance (EPV), Pharmacoenvironmentology and Pharmacovigilance in herbal medicines. In addition, data from observational epidemiological studies are playing an increasingly important role.
12th Global Pharmacovigilance & Drug Safety Summit, July 9-10, 2018 Sydney, Australia; 9th World Congress on Bioavailability & Bioequivalence,March 19-21, 2018 Dubai,UAE; 5th Annual Congress on Chemistry in Drug Discovery & Designing ,April 16-17, 2018 Dubai ,UAE, 2nd World Heart Congress May 14-16, 20178 Tokyo Japan; 9th Asian Biosimilars Congress August 20-22, 2018 Tokyo Japan .
Related Associations or Societies:
International Society for Pharmacoepidemiology Center for Drug Safety and Effectiveness Center for Public Health and Human Rights Center for Health Services and Outcomes Research International Pharmaceutical Federation International Society of Pharmacovigilance .
Pharma Risk Management was designed as a set of Pharmacovigilance activities and interventions designed to identify, characterise, prevent or minimise risks of medicinal and therapeutic products including the assessment of the effectiveness of their clinical reactions, interventions and combination therapies. Drug industry need to promote companies in Pharmacovigilance practice to use information technology and provide efficient software to implement the risk minimisation plans in drug development and manufacture. Pharmaceutical risk management monitors unlicensed, off labels and orphan drugs ,risk management labelling, regulatory decisions, legal protection, intellectual property, enforcement, marketing, and company reputation.
12th Global Pharmacovigilance & Drug Safety Summit, July 9-10, 2018 Sydney, Australia; 9th World Congress on Bioavailability & Bioequivalence,March 19-21, 2018 Dubai,UAE; 5th Annual Congress on Chemistry in Drug Discovery & Designing ,April 16-17, 2018 Dubai ,UAE, 2nd World Heart Congress May 14-16, 20178 Tokyo Japan; 9th Asian Biosimilars Congress August 20-22, 2018 Tokyo Japan .
Related Associations or Societies:
International Society for Pharmacoepidemiology Center for Drug Safety and Effectiveness Center for Public Health and Human Rights Center for Health Services and Outcomes Research International Pharmaceutical Federation International Society of Pharmacovigilance
Track 14: Regulatory Affairs:
Regulatory affairs is developed to protect public health by controlling the safety and efficacy of medical devices and pharmaceutical drug products in areas including pharmaceuticals, veterinary medicines, medical devices, pesticides, agrochemicals, cosmetics and complementary medicines. Regulatory affairs (RA), also called government affairs, are a profession within regulated industries, such as pharmaceuticals, medical devices, energy, banking, telecom etc.
12th Global Pharmacovigilance & Drug Safety Summit, July 9-10, 2018 Sydney, Australia; 9th World Congress on Bioavailability & Bioequivalence,March 19-21, 2018 Dubai,UAE; 5th Annual Congress on Chemistry in Drug Discovery & Designing ,April 16-17, 2018 Dubai ,UAE, 2nd World Heart Congress May 14-16, 20178 Tokyo Japan; 9th Asian Biosimilars Congress August 20-22, 2018 Tokyo Japan .
Related Associations or Societies:
The global Pharmacoepidemiology and clinical research market has been thought to purview USD 14.2 billion in 2016-2017 and is calculated to reach around USD 22 billion by the year 2021, growing at a CAGR (compounded annual growth rate) of 7.5%, during the forecast period 2016 to 2021. Clinical research denotes Clinical trial as a branch of clinical research that follows a standardized protocol. Clinical research is especially performed to estimate safety and efficacy of the advanced drug. Clinical trial data is indispensable for further approval of the drug and to import it into the market.
Clinical research is one of the basic technologies to precipitate the revolution of the entire country-Malaysia into a knowledge based economy and an industrialized nation by year 2020.
This sector is predicted to be upto worth US $1.37 million, and is expected to rise at a CAGR of 10.5%.
The United States and Canada have the apical market share in the Clinical research and clinical trials market, followed by Europe where Germany leads the market pursued by Poland and Western Europe. Asia is one of the quickest growing markets. India, China, Singapore and South Korea are the extensive players involved in this market. The Asia-Pacific region has turn into a beautiful destination for companies contributing CTMS software. The rise in this regional segment can be connected to a number of factors, such as rising number of clinical trials, existence of a broad number of CROs, government initiatives, boost in outsourcing of clinical trials, availability of large and diverse patient population, presence of less stringent regulatory guidelines as correlated to developed nations, and cost advantages offered by various countries.
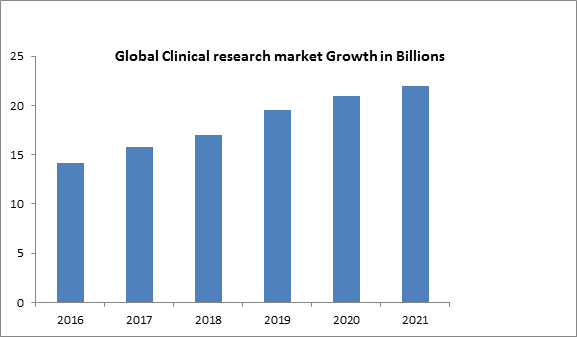
Leading companies are outsourcing their clinical trials to the APAC region due the lower cost of the clinical trials in the region as compared to the developed regions (Europe and North America).This high growth is attributed to the fact that the cost involved in performing a trial is less and the huge domain it provides in the aspect of diseases and patients.
The increasing disease burden is propelling the pharmaceutical and biotechnology industry to bring up new chemical entities into the market at a higher rate. Most companies are outsourcing the clinical trials of their newly developed drugs to various contract research organizations as this could save them the hassles of regulatory issues and patient recruitment burden from the research and development phase. Pharmacoepidemiology and Clinical research the drug to be tested for safety by different ethnic population. Due to the higher medical needs and increasing disease prevalence, developing countries are becoming a hub for clinical trial execution. Since 2006, there has been an increased number of trials registered worldwide and it is estimated to increase further in the coming years, which would contribute to the market growth.
The report analyzes the various trends and key opportunities in clinical trials market, and provides the market share of leading segments and geographical parts. This report contributes strategic insights on industry competition, and disguise key facts, business overview, service portfolio, SWOT scrutiny and financial analysis of leading players in the market. The primary segments subsidizing the growth strategies in global market of Clinical research are:
· eClinical solution vendors
· Clinical research organizations
· Pharmaceutical/biopharmaceutical companies
· Research and development (R&D) companies
· Business research and consulting service providers
· Medical research laboratories
· Academic medical centers/universities/hospitals
· Healthcare IT service providers
Pharmaceutical Societies and Associations across the world:
Center for Drug Safety and Effectiveness
Center for Public Health and Human Rights
Center for Health Services and Outcomes Research
European federation on Pharmaceutical sciences
Pharmaceutical Research and Manufacturers of America
National Pharmaceutical Association
Association of Pharmacy Professionals
Pharmaceutical Society of Ireland
The Pharmaceutical Association of Israel
Kuwait Pharmaceutical Association
Pharmaceutical Society Of New Zealand
Pharmaceutical Association of Mauritius
International Pharmaceutical Federation (FIP)
European Pharmaceutical Union (EPU)
Pharmaceutical Group of the European Union (PGEU)
European Association of Employed Community Pharmacists in Europe (EPhEU)
Australian College of Pharmacy
Pharmaceutical Society of Australia
The Pharmacy Guild of Australia
Canadian Pharmacists Association
Chinese Pharmaceutical Association
Further information: Pharmacy in China
Danish Association of Pharmaconomists
Pharmaceutical Society of Northern Ireland
Royal Pharmaceutical Society (RPS)
National Pharmacy Association
American Association of Colleges of Pharmacy
American Pharmacists Association
American Society for Pharmacy Law
American Society of Consultant Pharmacists Foundation
American Society of Consultant Pharmacists (ASCP)
American Society of Health-System Pharmacists
Professional Compounding Centers of America
Iranian Pharmacoepidemiology
Drug Safety Association
Conference Highlights
- Pharmacoepidemiology and Clinical Research
- Pharmacotherapy and Drug Safety
- Action of Drugs
- Clinical Research
- Clinical Trials
- Pharmacology
- Pharmacoepidemiology And Public Health
- Genetic Epidemiology
- Drug Discovery and Drug Development
- Clinical Data Management
- Clinical Pharmacy
- Pharmacovigilance and its Significance
- Risk Management
- Regulatory Affairs
- Entrepreneurs Investment Meet
- Pharmacoeconomics & Outcomes Research
- Study Designs in Pharmacoepidemiology
- Impact of Pharmacoepidemiology
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | October 17-18, 2018 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | |||
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Advances in Pharmacoepidemiology & Drug Safety
- Journal of Clinical Research & Bioethics
- Journal of Clinical Trials
Abstracts will be provided with Digital Object Identifier by
















